
| CAS: | 27164-46-1 | Mf: | C14H15N8NaO4S3 |
|---|---|---|---|
| MW: | 226.23 | Specification: | ≥98% |
| Appearance: | White Crystalline Powder | Whatsapp: | +86 15202961574 |
| High Light: | CAS 27164-46-1 API Antibacterial,API Antibacterial cefazolin sodium,CAS 27164-46-1 Antibacterial Raw Material |
||
Antibiotic API CAS 27164-46-1 Cefazolin Sodium powder
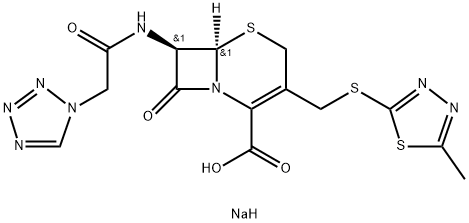
| Prodcut name | Cefazolin Sodium |
| Synonyms | Gramaxin;Cefazil;Cephazolin sodium;Cephazolin sodium salt;Azolin; |
| MOQ | 1KG |
| CAS | 27164-46-1 |
| Appearance | White powder |
| Molecular Formula | C14H13N8NaO4S3 |
![]()
What is Cefazolin Sodium?
Cefazolin Sodium is the first generation cephalosporin with a wide antibacterial spectrum. It is a white or almost white crystalline powder, odorless, bitter, easily soluble in water, slightly soluble in methanol, very slightly soluble in ethanol, insoluble in acet eer or chloroform.
Its antimicrobial spectrum is similar to cefexin, and it has antimicrobial activity against Staphylococcus (including enzyme producing strains), Streptococcus (except Enterococcus), Streptococcus pneumoniae, Escherichia coli, Proteus exotic, Claybiobacteria, Haemophilus influenzae and Enterobacter aerogenes.
The Application & Function of cefazolin Sodium
Mainly used in infections caused by sensitive bacteria or pathogens.
Mainly suitable for the treatment of otitis media, bronchitis, pneumonia and other respiratory tract infections, urinary tract infections, skin and soft tissue infections, bone and joint infections, sepsis, infective endocarditis, liver and biliary system infections and eye, ear, nose and throat infections caused by sensitive bacteria.
whatsapp:+86 152 0296 1574
Dose and method of administration
Cefazolin may be administered intramuscularly or intravenously after reconstitution. Total
daily dosag Intramuscular Administration:
Shake well until dissolved. The 500 mg vial can be reconstituted with 0.9% Sodium Chloride Injection, Sterile Water for Injection or Bacteriostatic Water for Injection.
ntravenous Administration:
Cefazolin may be administered by intravenous injection or by continuous or intermittent
The COA of Cefazolin Sodium
| Item | Specification | Results |
| APPEARANCE | white | CONFORMS |
| SOLUBILITY | FREELY SOLUBLE IN CHLOROFORM;SOLUBLE IN WATER OR METHANOL;INSOLUBLE IN ER | CONFORMS |
| PH | 5.0-7.2 | 6.4 |
| LOSS ON DRYING | ≤4.5% | 2.9% |
| RESIDUEON IGNITION | ≤2.5% | 0.2% |
| HEAVY METALS | ≤20PPM | <20PPM |
| TYRAMINE | ≤0.35% | 0.04% |
| RELATED COMPOSITIONS | TYLOSIN A ≥80% A+B+C+D ≥95% | 92% 97% |
| Conclusion | Confirm with the standard |